Responsible Conduct of Research (RCR) Training Program
On this Page
Description
Responsible Conduct of Research (RCR) training is required for undergraduate students, graduate students, postdoctoral trainees, research associates, staff, and faculty whose research is supported by certain federal funding agencies. RCR training reflects Penn’s commitment to research integrity, ethical scholarship, and compliance with sponsor and University requirements.
Projects funded by the National Institutes of Health (NIH), National Science Foundation (NSF), and the Department of Agriculture, National Institute of Food and Agriculture (USDA-NIFA) are subject to specific RCR training mandates. Required training may include online coursework, instructor-led seminars, or a combination of formats, depending on an individual’s school affiliation, career stage, and funding source.
Researchers and trainees should consult their faculty mentor or program administrator to confirm any additional, program-specific RCR expectations.
Core Components of RCR Training
- Research Misconduct
- Protection of Human Subjects
- Animal Welfare
- Contemporary Ethical Issues in Science
- Conflict of Interest and Commitment
- Peer Review
- Data Acquisition, Sharing, and Ownership
- Collaborative Research
- Publications Practice and Responsible Authorship
- Mentor and Trainee Relationships
Completing RCR Training via CITI
At Penn, required online RCR training is delivered through the Collaborative Institutional Training Initiative (CITI) platform and is assigned and tracked through Workday Learning.
How CITI Works at Penn
- RCR training assignments appear in Workday Learning.
- Training content is completed in the CITI system.
- Upon completion, training records are transmitted overnight to Workday.
- If completion does not appear after several business days, contact the Penn Solutions Center:
https://pennsolutioncenter.freshdesk.com/support/home
Step-by-Step: Completing RCR Training in CITI
Step 1: Log in Through Penn
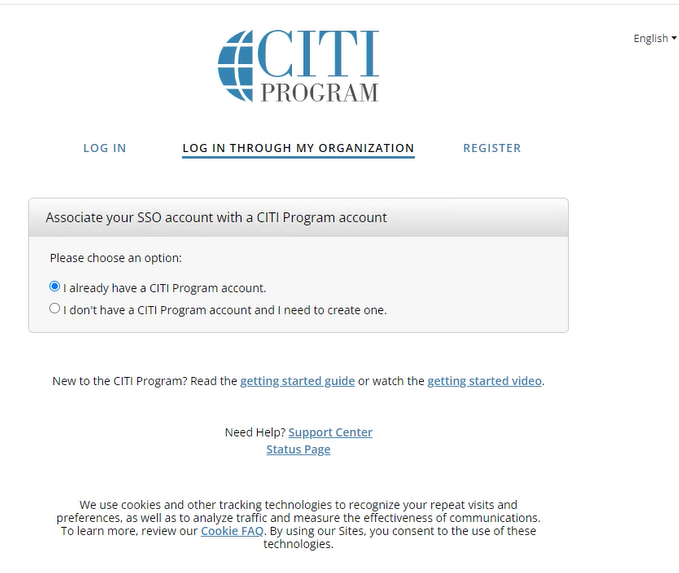
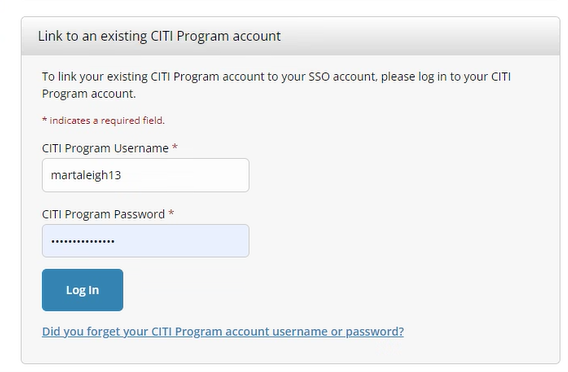
Go to the CITI Program website and select Log in through my organization. Log in using your PennKey.
If you already have a CITI account, select “I already have a CITI Program account” and enter your existing CITI username and password to link it to Penn. If you are new to CITI, select “I don’t have a CITI Program account” and follow the prompts to create one.
Step 2: Confirm Penn Affiliation / No Courses Assigned
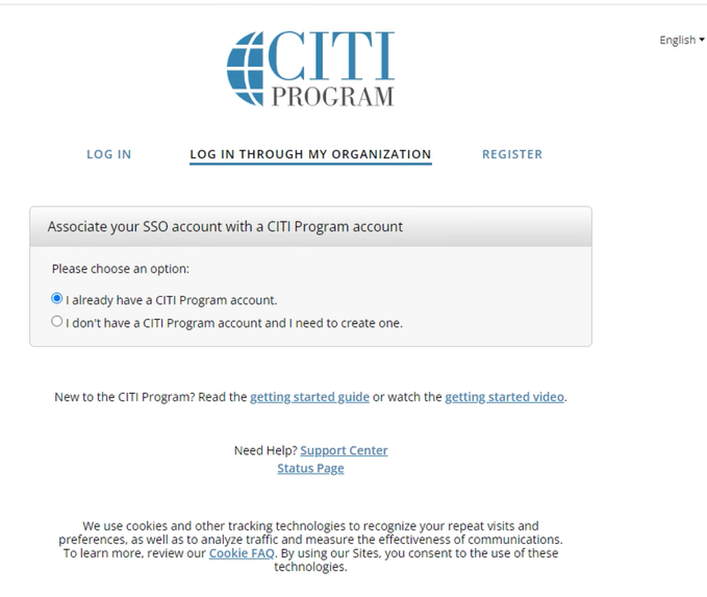
If you are not enrolled in any courses, confirm University of Pennsylvania and select Add a Course.
Next: Proceed to the course selection questions.
Step 3: Select the Correct RCR Course

Select “Responsible Conduct of Research (RCR) – For all other individuals, including NSF and NIH funded students and trainees.” Next: Click Next to choose your RCR subject area.
Step 4: Choose Your RCR Subject Area
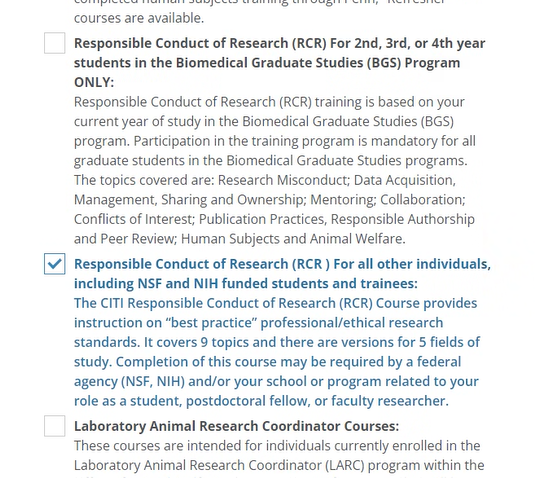
Choose one subject area that best aligns with your field.
Next: Confirm your selection to assign the required modules.
Step 5: Complete Assigned Modules
Select Start Now and complete all assigned modules.
Next: Finish all modules to record your completion.
Step 6: Verify Completion in Workday
After completing all modules, allow time for your training record to sync to Workday Learning.
Next: If completion does not appear after several business days, contact the Penn Solutions Center.
Additional Approved RCR Training Opportunities at Penn
In addition to CITI, Penn recognizes several program- and school-based RCR training options that may satisfy RCR requirements for specific populations or funding sources. These offerings are administered by their respective units.
- Biomedical Postdoctoral Programs (BPP) RCR Seminars
Instructor-led seminars open to all postdocs across disciplines, providing interactive, discussion-based Responsible Conduct of Research (RCR) training aligned with federal requirements. - Perelman School of Medicine: Responsible Conduct of Research (RCR) and Scientific Rigor and Reproducibility (SRR)
School-specific training that integrates RCR principles with NIH-aligned expectations for scientific rigor and reproducibility in biomedical research. - Principles of Responsible Conduct of Research
Core ethical principles underlying responsible research practice.
Researchers should confirm with their mentor, program, or school whether participation in these offerings satisfies their specific RCR training obligations.
Additional References and Learning Resources
The following resources provide authoritative guidance, case studies, and best practices related to the Responsible Conduct of Research. These materials are intended to supplement required RCR training and support ongoing learning, but do not replace Penn’s approved RCR training requirements unless explicitly stated.
- Office of Research Integrity (ORI) Introduction to the Responsible Conduct of Research. Foundational overview developed by the U.S. Department of Health and Human Services.
- National Institutes of Health (NIH) Data Management and Sharing Policy (2023)
Federal requirements and expectations for managing, sharing, and preserving research data. - National Institutes of Health (NIH)
Sponsor policies, guidance, and expectations related to research integrity. - National Institutes of Health (NIH) Responsible Conduct of Research (RCR) Training Policy
Sponsor policies, guidance, and expectations related to research integrity. - National Institutes of Health (NIH) Annual Review of Ethics (Case Studies)
Real-world case studies illustrating ethical challenges in research.
RCR Contacts
| Department / Program / School / Center | Contact | Website | |
| Biomedical Graduate Students (BGS) | Colleen Dunn | dunncoll@pennmedicine.upenn.edu | https://www.med.upenn.edu/bgs-rcr-exdes |
| Biomedical Postdoctoral Program Affiliates (BPP) (Perelman School of Medicine, School of Veterinary Medicine, School of Dental Medicine, School of Nursing, The Children’s Hospital of Philadelphia, Monell Chemical Senses Center, Swarthmore | Donna Copeland | postdoc@pennmedicine.upenn.edu | https://www.med.upenn.edu/postdoc/programs/rcr |
| School of Engineering and Applied Sciences (Graduate Students) | Alyse Edwards | aedwards@seas.upenn.edu | https://catalog.upenn.edu/courses/eas |
| School of Engineering and Applied Sciences (Postdoctoral Trainees) | Office of the Associate Dean for Research | adro@seas.upenn.edu | |
| School of Arts and Sciences (Graduate Students) | Division of Faculty Affairs (Janel Baselice) | faculty-affairs@sas.upenn.edu |